developed by: Richard A. Van Konynenburg, Ph.D
 Richard A. Van Konynenburg, Ph.D presented the Glutathione Depletion—Methylation Cycle Block Hypothesis for the pathogenesis of CFS in a poster paper at the 8th international conference of the International Association for Chronic Fatigue Syndrome in Ft. Lauderdale, Florida, on January 10-14, 2007. Due to subsequent requests from clinicians for a description of a treatment approach based on this hypothesis, Van Konynenburg came up with a Simple Approach and a more Customized Approach, based on what he perceives to be the most successful treatment approaches currently used in autism, which he believes shares the same basic pathogenetic mechanism with CFS. In most cases, it will be necessary for the physician to use the Customized Approach and tailor the treatment program to the individual patient. Van Konynenburg has since updated his approach and added some cautions. Please note that some people who have tried this approach have had adverse effects, sometimes of a serious nature.
Richard A. Van Konynenburg, Ph.D presented the Glutathione Depletion—Methylation Cycle Block Hypothesis for the pathogenesis of CFS in a poster paper at the 8th international conference of the International Association for Chronic Fatigue Syndrome in Ft. Lauderdale, Florida, on January 10-14, 2007. Due to subsequent requests from clinicians for a description of a treatment approach based on this hypothesis, Van Konynenburg came up with a Simple Approach and a more Customized Approach, based on what he perceives to be the most successful treatment approaches currently used in autism, which he believes shares the same basic pathogenetic mechanism with CFS. In most cases, it will be necessary for the physician to use the Customized Approach and tailor the treatment program to the individual patient. Van Konynenburg has since updated his approach and added some cautions. Please note that some people who have tried this approach have had adverse effects, sometimes of a serious nature.
Van Konynenburg believes that the fundamental biochemical issue in a large subset of the CFS patients is that the methylation cycle is blocked. The main goal of this treatment approach is to remove this block and restore the methylation cycle. He also believes that glutathione depletion is directly responsible for many of the features of CFS, but that it is usually not possible to normalize the glutathione levels on a permanent basis by direct approaches of glutathione supplementation. Rather, the methylation cycle block must be corrected first, to break the vicious circle that is holding down the glutathione levels. In addition to this, about one-third of CFS patients, because of particular genetic polymorphisms, cannot tolerate supplementation with glutathione or other substances intended to help them directly to build glutathione.
Based on what is being done in autism by the Defeat Autism Now! (DAN!) researchers and clinicians and independently by Dr. Amy Yasko, N.D., Ph.D., he suggests two treatment approaches for CFS. The first is a Simple Approach better suited to patients who have been ill a relatively short time and are not disabled. Use of this simplified approach would be based on the hope that the patient does not have certain genetic polymorphisms, which would not be known in this simplified approach.
If the patient does in fact have these polymorphisms, the simplified approach will not be successful, and the customized program should be followed. This simpler treatment approach is based partly on the treatment that was used by Dr. S. Jill James, Ph.D., et al. in the study that found the connection between the methylation cycle block and glutathione depletion in autism (Ref. 2 in his pathogenesis paper), but it makes use of supplements that are part of Dr. Amy Yasko’s treatment program.
The second treatment approach is much more involved and is based on Dr. Yasko’s complete autism treatment. Van Konynenburg believes that the second approach is the treatment that will be necessary for most CFS patients, especially those who are more debilitated/disabled, as well as those with certain genetic polymorphisms.
TREATMENT ISSUES
- It is necessary to minimize the use of pharmaceuticals in treating CFS patients. Many CFS patients have polymorphisms in their detox enzymes, including CYP450 enzymes and Phase II detox enzymes. (See Detoxigenomic panel offered by http://www.genovations.com). Because of these polymorphisms, many patients are genetically unable to detox pharmaceuticals at normal rates, and cannot tolerate them. In addition, all patients who have the glutathione depletion and methylation cycle block suffer from biochemical inhibition of their detox systems, whether they have these polymorphisms or not. Because of these two factors, CFS patients suffer from the toxic effects of pharmaceuticals. Treatment using nutritional supplements is necessary, and some herbals can be tolerated as well.
- Because of the broad nature of the current case definition for CFS, the population defined by it is very heterogeneous. It is likely that the pathogenesis model Van Konynenburg has presented for CFS will not fit all patients. For this reason, he recommends a relatively inexpensive glutathione measurement initially, such as the red blood cell total glutathione test offered by http://www.immuno-sci-lab.com (phone them for details) or by Mayo Laboratories. Perhaps a better test is the serum reduced glutathione test offered as part of the Comprehensive Detox Panel at http://www.gdx.net/home/assessments/detox/reports/. If a below-normal value is found in either of these tests, there may be a good chance that this pathogenesis model fits the patient.
- Different patients have different genetic polymorphisms in the enzymes and other proteins that impact the methylation cycle and the associated biochemical cycles and pathways. Some of these polymorphisms will have important impacts on the choice of specific parts of the treatment program. In using this more complicated treatment approach, it will be necessary to characterize the polymorphisms before it will be possible to make some of the decisions about selection of particular treatment aspects.
The most comprehensive panel for this is Dr. Yasko’s Comprehensive Basic SNP (single nucleotide polymorphism) Panel I, available from http://www.holisticheal.com. Dr. Yasko has selected the polymorphisms on this panel by correlating their presence with severity of autism symptoms and with the results of biochemical testing (mainly spot urine tests for organic acids, amino acids, and essential and toxic metals). This is a somewhat unorthodox method that jumps over the usual intermediate steps involved in studying polymorphisms, and there is not universal agreement about her results in the research community, but Dr. Yasko’s treatment outcomes are speaking for themselves, as can be seen from the voluntary testimonials of parents of autistic children on the parents discussion group at http://www.autismanswer.com.
The results from this genetic panel require interpretation. One can either study Dr. Yasko’s materials to gain her insights on interpreting the results in general, or order her interpretation of the particular results, which is called a Genetic Analysis Report or GAR. The GAR is a computer-generated report with some general material that applies to all the cases, and specific sections that are chosen in response to the particular genetic polymorphisms found in the individual patient. As such, the continuity of the discussion in the GAR is not what would be found in a report written from scratch for each particular patient, and it may have to be read more than once to make all the connections in one’s mind, but the material contained is specific to the particular genetic panel results, and Dr. Yasko updates the material used in generating the GARs as more is learned. - As discussed in Van Konynenburg’s paper, people who have been ill for an extended period of time (many months to many years) will have accumulated significant infections and significant body burdens of toxins, because both their cell-mediated immune response and their detox system will have been dysfunctional during this time. When the methylation cycle is then restarted, both the immune system and the detox system will begin to function better. When they do, pathogens and infected cells will begin to die off at higher rates, and toxins will be mobilized. The resulting detoxification will be unpleasant, and may even be intolerable. If the patient has not been prepared in certain ways, discussed below, she or he may not be willing to continue this and may drop out of the treatment program.
- One of the most important preparatory activities is to make sure the gastrointestinal system is operating well enough to be able to absorb nutrients, including both food and the oral supplements used in the treatment, and also well enough to be able to dispose of toxins into the stools on a regular basis. If this is not done, it is likely that the treatment will not be successful. Treatments for the G.I. system, as well as for other aspects described below, are discussed in Dr. Amy Yasko’s book. Some CFS patients have reported benefit from Xifaxan to treat deleterious bacteria in the gut. This antibiotic is not absorbed from the G.I. tract, so it does not present problems for the detox system.
- Another very important aspect of the preparation is to deal with the overstimulation or overexcitation of the nervous system that is present in CFS. This probably results from several causes, including depletion of magnesium and in some cases depletion of taurine, low blood flow to the brain because of low cardiac output, glutathione depletion in the brain producing mitochondrial dysfunction, and dietary and other factors causing elevation of excitatory neurotransmitters and depletion of inhibitory neurotransmitters. It is important that this be dealt with because if it is not, the patient will be less able to tolerate the detox inherent in the treatment.
- Another important step is to ensure that the patient’s nutritional status is supported. Many CFS patients are in a rather debilitated state, partly because of deficiencies of essential nutrients. They are also in a state of oxidative stress. Appropriate nutritional supplements can correct these problems at least to some degree and get the overall metabolism of the patient into a better state, so that they can better tolerate the detox part of the treatment.
- Particular organs or systems may not be functioning well and may need extra nutritional or herbal support. Which ones will vary from one patient to another, so this part of the treatment must be tailored to the individual patient.
- Chronic bacterial infections should be addressed. According to Dr. Yasko, females in particular appear to be prone to streptococcal infections. She also finds that aluminum appears to be associated with the bacteria, so that when the bacteria die off, aluminum is excreted. While antibiotics can be used, there are downsides to this, both in terms of difficulty in detoxing some of the antibiotics and in terms of killing beneficial intestinal flora and encouraging deleterious ones, such as Clostridia dificile. In addition, some CFS patients have experienced tendon problems from the fluoroquinolone antibiotics. Dr. Yasko prefers natural antimicrobial treatments.
- When the methylation cycle is restored, the normal detox system is able to deal with more of the toxins. Dr. Yasko also uses low doses of oral EDTA, but not the sulfur-containing chelators (DMSA and DMPS), to help remove aluminum as well as other metals, including mercury. DMSA and DMPS are not used because they can also bind glutathione, so that if a patient who is low in glutathione receives these chelators, their glutathione status can be worsened. Also, DMSA and DMPS are rich in sulfur, and CFS patients with certain polymorphisms cannot tolerate them. She also uses some natural RNA formulas for detoxing, as well as for a number of other purposes during the treatment. These are somewhat costly, and are not required as part of the treatment, but are reported to be helpful.
- As mentioned in item 3 above, it is important to characterize relevant polymorphisms prior to bringing up the methylation cycle operation. One of the most important aspects of this is to evaluate polymorphisms in the CBS (cystathionine beta synthase) enzyme, which is located at the entrance to the transsulfuration pathway and converts homocysteine to cystathionine. Although this is somewhat controversial within the research community, Dr. Yasko finds that certain polymorphisms cause an increase in the activity of this enzyme. The result is that there is too large a flow down the transsulfuration pathway, and somewhat counter intuitively this results in lowered production of glutathione, as well as elevated production of taurine, ammonia, sulfite and hydrogen sulfide. The last three of these substances are toxins. If a patient has CBS polymorphisms, it is necessary to deal with this aspect before restarting the methylation cycle. If this is not done, efforts to start this cycle will result in increased production of these toxins. This may explain why some patients cannot tolerate direct efforts to build glutathione using sulfur-containing substances, while others derive some benefit from this. Dealing with this CBS up regulation situation can take a month or longer.
- Only after all these issues have been addressed is the patient ready to start supplementing with larger amounts of the folates and cobalamins to begin major restoration of operation of the methylation cycle.
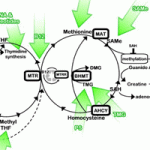
- As you can see from the diagram in Van Konynenburg’s pathogenesis paper, there are two possible pathways from homocysteine to methionine. One involves the enzyme methionine synthase, which requires methylcobalamin and is linked to the folate cycle as well, and the other involves the enzyme betaine homocysteine methionine transferase (BHMT), and requires trimethylglycine or one of the phospholipids (phosphatidyl-serine, -choline, or -ethanolamine). Ultimately, it is important to get the methionine synthase pathway back into operation, but in Dr. Yasko’s practice it has been found that it is easier to start up the BHMT pathway first. Van Konynenburg thinks the reason is that S-adenosylmethionine (SAMe) interacts with methionine synthase, and by first starting up the BHMT pathway, one ensures that there is enough SAMe to start up the methionine synthase pathway.
- As these steps are taken, the immune system and the detox system will start to function at higher levels, and die-off and detox will begin. These processes are monitored using periodic spot urine testing, and decisions about when to proceed to the next step in the treatment program are based on this urine testing.
- Viral infections are dealt with naturally as the immune system recovers, though Valtrex is used in some cases. As the viruses die off, it is observed that heavy metal excretion increases. Heavy metal excretion is tracked using periodic spot urine tests and is plotted as a function of time to determine the progress.
- When appropriate indications are seen in the urine testing, the BHMT pathway is slowed using dimethylglycine, which is a product of the BHMT reaction, and thus exerts product inhibition on it. This shunts the flow through the parallel methionine synthase pathway. This has the effect of bringing up the folate cycle, which is linked to it, and also bringing up the biopterin cycle, which is linked to the folate cycle. The folate cycle is needed to make new RNA and DNA to proliferate new cells, such as T cells in cell-mediated immunity. The biopterin cycle is necessary for the synthesis of serotonin and dopamine as well as for the operation of the nitric oxide synthases. Some patients benefit from direct supplementation of tetrahydrobiopterin, often in very small amounts.
- The treatments up to this point should resolve most of the symptoms of CFS. The last step is to support remyelination, which has been dysfunctional during the time when the methylation cycle was blocked, because methylation is necessary to synthesize myelin basic protein. This should improve the operation of the nervous system.
That is a rough outline of the treatment process. Please see Dr. Yasko’s materials for the details.
This treatment approach is not simple, quick, easy or inexpensive, but Van Konynenburg thinks this rather complex process is what is required. He would very much appreciate it if you decide to try this treatment approach, that you will keep him informed of how it works out for your patients. He is happy to answer questions that come up as well.
A yahoogroup has been started for those wanting to explore Dr. Amy Yasko’s protocol and Rich Van Konynenburg’s treatment plans in depth: http://health.groups.yahoo.com/group/CFS_Yasko/. Please note, this is a treatment group and not a support group.
see also: Dr. Amy Yasko’s Nutrigenomics Protocol
source(s): Suggestions for Treatment of Chronic Fatigue Syndrome (CFS): The Simple Approach, the Glutathione Depletion—Methylation Cycle Block Hypothesis for the Pathogenesis of CFS by Richard A. Van Konynenburg, Ph.D.








I emailed Rich Van Konynenburg to see how he felt about using this protocol for MCS and this was his response:
The treatment is premised on the presence of glutathione depletion and a partial block in the methylation cycle. My hypothesis for MCS is that glutathione is depleted in the sustentacular cells of the olfactory epithelium in the “ceiling” of the nasal cavity. In cases where this is true, I think this treatment will help, because it restores glutathione levels. I think this is the mechanism involved when MCS is associated with CFS.
There may be another subset of MCS that does not involve glutathione depletion. For example, in cases in which the MCS began with a large exposure of some chemical, I think the mechanism might be different, such as involving damage to the olfactory epithelium which is not repaired.
Did your MCS start with a large acute exposure to some chemical?
One way to tell for sure whether your glutathione is depleted is to take the Vitamin Diagnostics, Inc., methylation pathways panel. Here is the contact information:
Methylation Pathways Panel
This panel will indicate whether a person has a partial methylation cycle block and/or glutathione depletion. I recommend that this panel be run before deciding whether to consider treatment for lifting the methylation cycle block. I am not associated with the lab that offers this panel.
The panel costs $300 and requires an order from a physician or a chiropractor. The best way to order the panel is by fax, on your clinician’s letterhead.
Available from:
Vitamin Diagnostics, Inc.
Rt. 35 & Industrial Drive
Cliffwood Beach, NJ 07735
USA
Phone:+1 (732) 583-7773
Fax: +1 (732) 583-7774)
Lab Director: Tapan Audhya, Ph.D.
(usually at the lab on Tues. and Wed. from 1 to 3 p.m., Eastern time)
Dr. Audhya is willing to help clinicians with interpretation of the panel by phone, or you can interpret the panel results using the following information:
Interpretation of the Vitamin Diagnostics Methylation Pathways Panel by Rich Van Konynenburg, Ph.D.
Several people have asked for help in interpreting the results of their Vitamin Diagnostics, Inc., methylation pathway panels. Here are my suggestions for doing so. They are based on my study of the biochemistry involved, on my own experience with interpreting more than 120 of these panel results to date, and on discussion of some of the issues with Tapan Audhya, Ph.D., who is the director of the Vitamin Diagnostics lab.
The panel consists of measurement of two forms of glutathione (reduced and oxidized), adenosine, S-adenosylmethionine (SAM) , S-adenosylhomocysteine (SAH), and seven folic acid derivatives or vitamers.
According to Dr. Audhya, the reference ranges for each of these metabolites was derived from measurements on at least 120 healthy male and female volunteer medical students from ages 20 to 40, non- smoking, and with no known chronic diseases. The reference ranges extend to plus and minus two standard deviations from the mean of these measurements.
Glutathione: This is a measurement of the concentration of the reduced (active) form of glutathione (abbreviated GSH) in the blood plasma. From what I’ve seen, most people with chronic fatigue syndrome (PWCs) have values below the reference range. This means that they are suffering from glutathione depletion. As they undergo the simplified treatment approach to lift the methylation cycle block, this value usually rises into the normal range over a period of months. I believe that this is very important, because if glutathione is low, vitamin B12 is likely unprotected and reacts with toxins that build up in the absence of sufficient glutathione to take them out. Vitamin B12 is thus “hijacked,” and not enough of it is able to convert to methylcobalamin, which is what the methylation cycle needs in order to function normally. Also, many of the abnormalities and symptoms in CFS can be traced to glutathione depletion.
Glutathione (oxidized): This is a measurement of the concentration of the oxidized form of glutathione (abbreviated GSSG) in the blood plasma. In many (but not all) PWCs, it is elevated above the normal range, and this represents oxidative stress.
Adenosine: This is a measure of the concentration of adenosine in the blood plasma. Adenosine is a product of the reaction that converts SAH to homocysteine. In some PWCs it is high, in some it is low, and in some it is in the reference range. I don’t yet understand what controls the adenosine level, and I suspect there is more than one factor involved. In most PWCs who started with abnormal values, the adenosine level appears to be moving into the reference range with methylation cycle treatment, but more data are needed.
S-adenosymethionine (RBC) (SAM): This is a measure of the concentration of SAM in the red blood cells. Most PWCs have values below the reference range, and treatment raises the value. S-adenosylmethionine is the main supplier of methyl groups in the body, and many biochemical reactions depend on it for their methyl groups. A low value for SAM represents low methylation capacity, and in CFS, it appears to result from a partial block at the enzyme methionine synthase. Many of the abnormalities in CFS can be tied to lack of sufficient methyation capacity.
S-adenosylhomocysteine (RBC) (SAH): This is a measure of the concentration of SAH in the red blood cells. In CFS, its value ranges from below the reference range, to within the reference range, to above the reference range. Values appear to be converging toward the reference range with treatment. SAH is the product of reactions in which SAM donates methyl groups to other molecules.
Sum of SAM and SAH: When the sum of SAM and SAH is below 268 micromoles per deciliter, it appears to suggest the presence of upregulating polymorphisms in the cystathione beta synthase (CBS) enzyme, though this may not be true in every case.
Ratio of SAM to SAH: A ratio less than about 4.5 also represents low methylation capacity. Both the concentration of SAM and the ratio of concentrations of SAM to SAH are important in determining the methylation capacity.
5-CH3-THF: This is a measure of the concentration of 5-methyl tetrahydrofolate in the blood plasma. It is normally the most abundant form of folate in the blood plasma. It is the form that serves as a reactant for the enzyme methionine synthase, and is thus the most important form for the methylation cycle. Many PWCs have a low value, consistent with a partial block in the methylation cycle.
The simplified treatment approach includes FolaPro, which is commercially produced 5-CH3-THF, so that when this treatment is used, this value rises in nearly every PWC. If the concentration of 5-CH3-THF is within the reference range, but either SAM or the ratio of SAM to SAH is below the reference values, it suggests that there is a partial methylation cycle block and that it is caused by unavailability of sufficient bioactive B12, rather than unavailability of sufficient folate. I have seen this frequently, and I think it demonstrates that the “hijacking” of B12 is the root cause of most cases of partial methylation cycle block. Usually glutathione is low in these cases, which is consistent with lack of protection for B12, as well as with toxin buildup.
10-Formyl-THF: This is a measure of the concentration of 10-formyl tetrahydrofolate in the blood plasma. It is usually on the low side in PWCs. This form of folate is involved in reactions to form purines, which form part of RNA and DNA as well as ATP.
5-Formyl-THF: This is a measure of the concentration of 5-formyl tetrahydrofolate (also called folinic acid) in the blood plasma. Most but not all PWCs have a value on the low side. This form is not used directly as a substrate in one-carbon transfer reactions, but it can be converted into other forms of folate. It is one of the supplements in the simplified treatment approach, which helps to build up various other forms of folate.
THF: This is a measure of the concentration of tetrahydrofolate in the blood plasma. In PWCs it is lower than the mean normal value of 3.7 nanomoles per liter in most but not all PWCs. This is the fundamental chemically reduced form of folate from which several other reduced folate forms are made. The supplement folic acid is converted into THF by two sequential reactions catalyzed by dihydrofolate reductase (DHFR). THF is also a product of the reaction of the methionine synthase enzyme, and it is a reactant in the reaction that converts formiminoglutamate (figlu) into glutamate. If figlu is high in the Genova Diagnostics Metabolic Analysis Profile, it indicates that THF is low.
Folic acid: This is a measure of the concentration of folic acid in the blood plasma. Low values suggest folic acid deficiency in the current diet. High values are sometimes associated with inability to convert folic acid into other forms of folate, such as because of polymorphisms in the DHFR enzyme. They may also be due to high supplementation of folic acid.
Folinic acid (WB): This is a measure of the concentration of folinic acid in the whole blood. See comments on 5-formyl-THF above. It usually tracks with the plasma 5-formyl-THF concentration.
Folic acid (RBC): This is a measure of the concentration of folic acid in the red blood cells. The red blood cells import folic acid when they are initially being formed, but during most of their approximately four-month life, they do not normally import, export, or use it. They simply serve as reservoirs for it, giving it up when they are broken down. Many PWCs have low values. This can be caused by a low folic acid status in the diet over the previous few months, since the population of RBCs at any time has ages ranging from zero to about four months. However, in CFS it can also be caused by damage to the cell membranes, which allows folic acid to leak out of the cells. Dr. Audhya reports that treatment with omega-3 fatty acids can raise this value over time.